Tags
Policy to plate: What genome-edited rice means for India’s food future
The release of genome-edited rice marks a policy and scientific shift, positioning India to lead biotech-driven farming amid climate, resource, and food security challenges.

CD Mayee / Bhagirath Choudhary
In a landmark scientific breakthrough in agriculture, the Government of India has officially released the world’s first two genome-edited rice varieties developed using the CRISPR-Cas9 technique, which marks a transformative step in its policy on agricultural biotechnology. The two rice varieties — DRR Dhan 100 (Kamla), developed by ICAR–Indian Institute of Rice Research (IIRR), Hyderabad, and Pusa Rice DST 1, by the ICAR–Indian Agricultural Research Institute (IARI), New Delhi — represent India’s first genome-edited crops to receive public approval.
This milestone is being celebrated by scientists and farmers alike as India’s first major success in precision breeding using genome editing. It is particularly significant considering India’s historically cautious approach to genetically modified (GM) crops. Since the release of Bt cotton in 2002, agricultural biotechnology has faced multiple hurdles, including a moratorium on Bt brinjal in 2010, delays in approving GM mustard, and stalled next-generation Bt/HT cotton technologies. These were compounded by policy bottlenecks such as the requirement for state-level NOCs for field trials, high testing costs, and a non-functional Event-Based Approval Mechanism (EABM). Adding to the challenges is the decade-long enforcement of the Cotton Seeds Price (Control) Order, 2015, which mandates a fixed maximum retail price (MRP) for Bt cotton seeds and further discouraged private investment and biotech-based innovation in the sector. Against this backdrop, the approval of genome-edited rice marks a strategic and science-backed policy shift aligned with global best practices.
How is Genome Editing Different from Genetic Modification?
Unlike genetically modified organisms (GMOs), the new rice lines contain no foreign DNA. Instead, scientists used the CRISPR-Cas9 system under the SDN-1 approach to make precise changes in native genes, enabling traits such as higher yield and drought and salinity tolerance without the regulatory complications of genetic modification or transgenics. Although transgenes were used in the development phase, the final products are free from foreign DNA. This development underscores the rising importance of CRISPR-based precision breeding in modern agriculture. Genome editing, particularly through the SDN-1 and SDN-2 pathways, allows for targeted, predictable changes in an organism’s DNA without introducing any foreign genetic material — a key distinction that has opened doors to regulatory flexibility and public acceptance.
Comparing DRR Dhan 100 (Kamla) and Pusa Rice DST 1
Among the two, the DRR Dhan 100 ‘Kamla’ variety is built on the widely cultivated Samba Mahsuri background and stands out for its innovation. IIRR researchers used a novel OsCKX2-deficient mutant allele, modified through SDN-1 genome editing to increase cytokinin levels in the rice panicle tissue. The loss of OsCKX2, a gene in rice that encodes a cytokinin oxidase enzyme involved in the degradation of cytokinin, thus boosts the growth-promoting cytokinin hormone in rice panicle tissue, resulting in higher grain yield and better productivity.
On the other hand, the Pusa Rice DST 1 variety was developed by ICAR-IARI in the popular MTU1010 rice background by editing the DST gene using the SDN-1 technique of CRISPR-Cas9. By knocking out a gene responsible for suppressing stress resistance, again using SDN-1 technology, the scientists achieved plants with reduced stomatal density and water use, alongside improved tillering, grain yield, and salt tolerance.
Both varieties were tested under the All India Coordinated Research Project (AICRP) on Rice and showed significantly better performance under drought and salinity stress compared to their parent varieties. Benefits include early maturity, drought tolerance, increased productivity (up to 15–20 per cent), reduced production costs, and better climate adaptability. Cultivating these improved varieties over five million hectares in eastern and southern India could yield 4.5 million tonnes of extra paddy and save about 7,500 million cubic metres of irrigation water, while reducing greenhouse gas emissions by 20 per cent, as estimated by ICAR.
Building a Regulatory Framework for Genome Editing
India regulates genome-edited and GM crops under the Rules for the Manufacture, Use, Import, Export and Storage of Hazardous Microorganisms, Genetically Engineered Organisms or Cells, 1989 (Rules 1989) of the Environment (Protection) Act (EPA), enforced by the Ministry of Environment, Forest and Climate Change (MoEF&CC) and coordinated with the Department of Biotechnology (DBT).
While upstream research is regulated by the Institutional Biosafety Committee (IBSC) and the Review Committee on Genetic Manipulation (RCGM) under the supervision of the Department of Biotechnology (DBT), Ministry of Science and Technology, downstream R&D, field trials, and environmental release are regulated by the Genetic Engineering Appraisal Committee (GEAC) of MoEF&CC. The broad regulatory framework of GM crops and genome-edited plants is a perfect example of co-development and regulation jointly by multiple relevant ministries based on their expertise.
The process began in 2020, when DBT initiated consultations on genome editing guidelines. The resulting “Guidelines for the Safety Assessment of Genome Edited Plants” incorporate gene technologies like self-cloning, gene deletion, and cell hybridisation — thereby ensuring comprehensive regulation of research, development, import, manufacture, and storage of genome-edited plants. The guidelines classified genome editing into three categories, in which both SDN-1 and SDN-2 introduce small changes without adding any foreign DNA, while SDN-3 is typically categorised as transgenic and falls under more stringent biosafety and regulatory scrutiny, similar to GM technology.
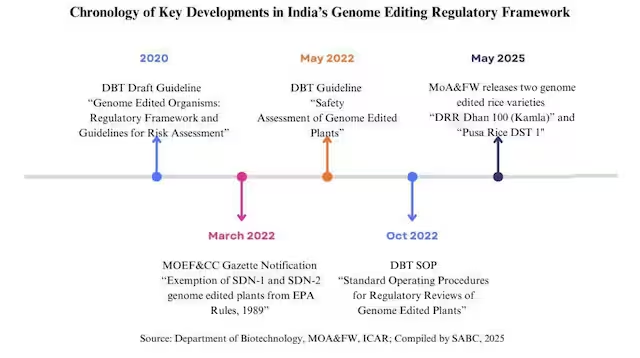
Dispelling the Myth of Regulatory Exemption of Genome-edited Plants
Contrary to claims, SDN-1 and SDN-2 genome-edited plants are not exempt from GM regulation. Initial oversight under the EPA Rules 1989 applies if foreign gene, DNA, or vector sequences are present. Once these elements are proven absent by the Review Committee on Genetic Manipulation (RCGM), the products are no longer considered transgenic and exit the EPA Rules 1989 regulatory framework via Rule 20. In contrast, genome-edited plants developed using the SDN-3 category containing foreign genes are subjected to further regulatory scrutiny similar to that of GM crops.
Following this, SDN-1 and SDN-2 genome-edited plants undergo varietal evaluation under the Seeds Act, 1966, Seeds Rules, 1968, and the Seeds (Control) Order, 1983, administered by the Ministry of Agriculture and Farmers’ Welfare. This involves multi-location trials under ICAR-AICRP, followed by registration and release by the Central Varietal Release Committee (CVRC). This dual-track regulation — initial biosafety checks followed by agronomic evaluation — is both scientifically sound and time-bound.
The term “exemption” refers specifically to Rule 20 of the EPA Rules, which also allowed rDNA pharma products to be regulated separately from GMOs after a 2006 task force led by RA Mashelkar. Those pharma products are now regulated by IBSC, RCGM, and then by the Drugs Controller General of India (DCGI) under the Ministry of Health and Family Welfare.
By applying a similar, streamlined regulatory approach to SDN-1 and SDN-2 genome-edited plants, India aims to avoid the protracted bureaucratic hurdles that have historically delayed the commercialisation of GM crops. The streamlined framework eliminates the need for cumbersome state-level NOCs, enabling faster and more predictable crop development.
The successful release of these genome-edited rice varieties marks a turning point — not just in science, but in policy on GM crops. It reflects a new era of biotech-driven agriculture tailored to meet the challenges of climate change, resource scarcity, and the growing food demands of a burgeoning population. India is now poised to lead in genome-edited crop innovation and agricultural biotechnology.
https://www.business-standard.com/economy/analysis/policy-to-plate-what-genome-edited-rice-means-for-india-s-food-future-125051100608_1.htmlPublished Date: May 11, 2025






